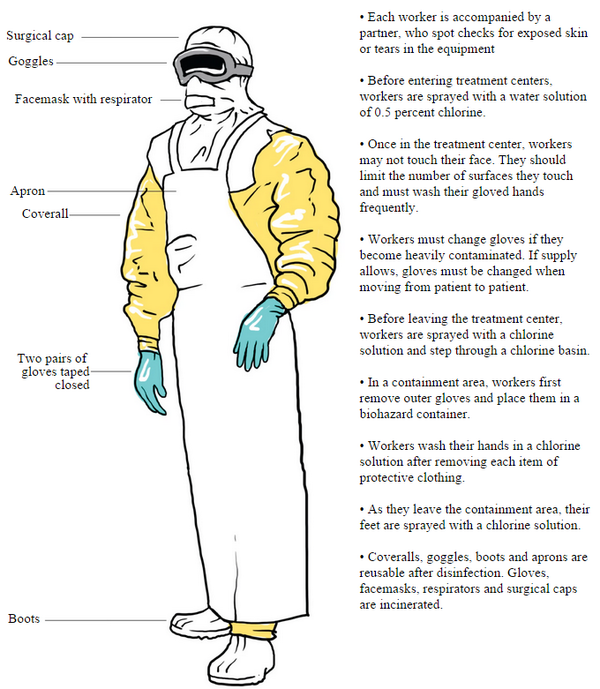
Sorry but D'uh - yes the numbers during the Ebola virus disease (EVD) outbreak happening since December in Guinea then progressing to Sierra Leone, Liberia, Nigeria and Senegal....are an underestimate.
Of course they are!
How could they possibly not be?
Have you not watched a single documentary or news video detailing how heartbreakingly difficult it is to visit and help the people of West Africa, to characterize and gather those case numbers, to take, transport and test samples?
The suspect cases are an underestimate.
The probable cases are an under-estimate.
The fatal cases are an under-estimate.
The only thing that is spot on is the laboratory confirmation numbers, because they are what they were when someone wrote them down having had some semblance of control over the steps to acquire them.
But let's put that underestimation into context.
 |
| "The tip of the iceberg" Image originally provided by Gregory Haertl, WHO. Click to enlarge |
In fact, some of those, the subtyping numbers, are deliberately so because it's too expensive and wasteful to subtype every single laboratory confirmed case - so a sample of cases are tested and that is assumed to reflect the subtype distribution for that region during that period.
But seasonal influenza case numbers as a whole are a huge underestimate. Influenza does not drive everyone to a general practitioner nor to a hospital. Some infections with influenza virus don't even produce noticeable symptoms at all. They are still infections. They just don't get counted. So influenza A virus, possibly the most tracked of any respiratory virus, is underestimates. And that's okay.
Well, measles too, in the respiratory virus department.
The latest big bad is the species D enterovirus 68 (EV-D68). But the paltry few detections of it (identified by genotyping) that have reported across the United States are likely a monstrous underestimate. In fact we have very little idea of a normal denominator for EV-D68 detections so it's hard to even know if 2014 is seeing all that big a change in its spread and distribution. Usually the enteroviruses (includes rhinoviruses) cause common cold-like illnesses and only get sought out in the great detail from a research point of view.
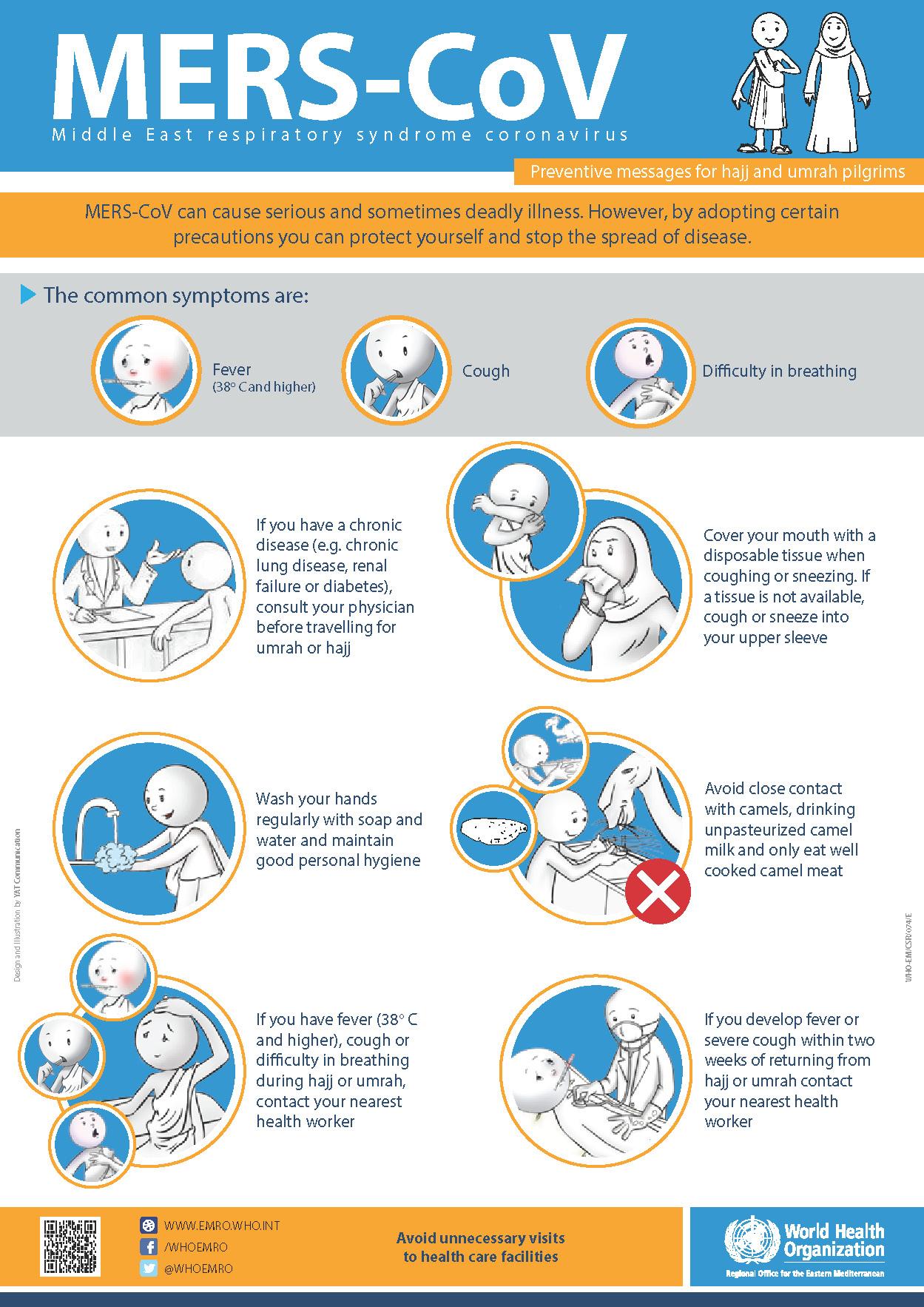
Middle East respiratory syndrome coronavirus (MERS-CoV) cases or the emerging influenza A(H7N9) virus cases are all underestimated as well.
The population of your state or country is an underestimate too you know?
This is because we cannot capture every single case of infection, or person, at once.
So the next time you are about to say "the WHO numbers are an underestimate" as if that is a revelation or an unexpectedly horrible thing you can also lay at their doorstep - please just don't. It's not smart, new or unusual.
You might as well say the world is round; underestimation of infection numbers is just that well established a fact. It's just by how much, and frankly that doesn't even matter too much because the trends can usually be easily seen, or quickly extrapolated.
Perhaps you did not know all that before. But if you have read to here, you do now.